Arbutus menziesii
Sponsor
Kindly sponsored by
a member of the International Dendrology Society
Credits
Julian Sutton (2021)
Recommended citation
Sutton, J. (2021), 'Arbutus menziesii' from the website Trees and Shrubs Online (treesandshrubsonline.
Genus
Common Names
- Pacific Madrone
- Madrone
- Madrona
- Arbousier d'Amérique
Synonyms
- Arbutus procera Douglas
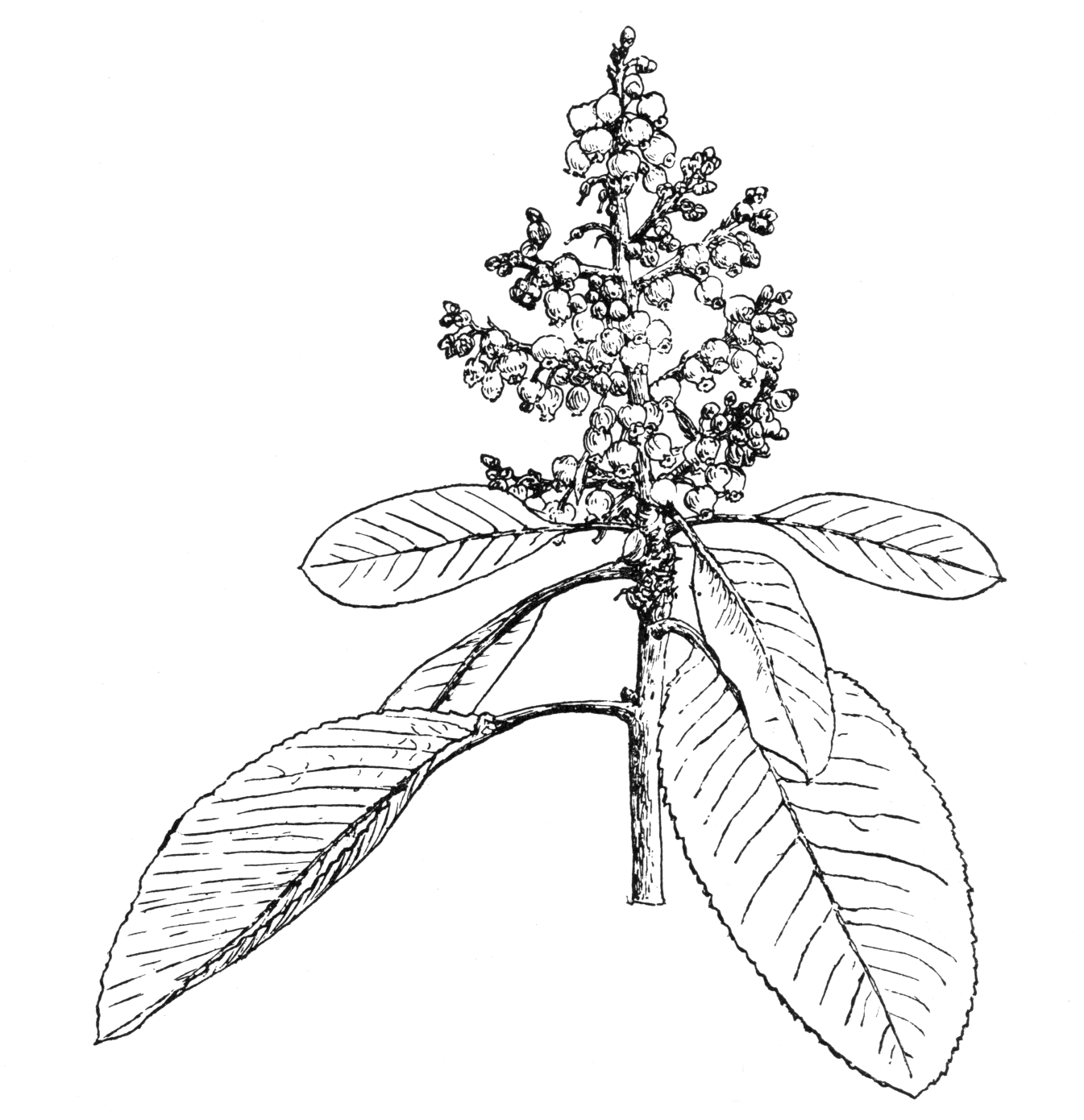
Shrub or tree, 4–10(–30) m. Bark dark red, smooth until mid-season when it exfoliates, revealing initially green inner bark; in older trees, bark retained (dark grey) on older parts of trunk and undersides of major branches. Leaves elliptic, 6.5–13 × 3.5–6(–8) cm, base usually rounded, apex rounded or acute, margins entire (sometimes serrate on vigorous sprouts), olive green green above, glaucous beneath, both surfaces sparsely hairy at first; petiole 2–4.5 cm, glabrous. Inflorescence a cluster of racemes, sometimes dense; pedicels sparsely hairy or glabrous, pendulous at first, erect later, 3–5 mm in flower, lengthening to 6–8 mm in fruit. Corolla urceolate, 5–6 mm, dull white; flowering March-May in the wild (May in Britain). Fruits rounded, 13–20 mm across, ripening to red or orange-red in July-September, sometimes retained into the New Year in the wild. (Sørensen 2009; Bean 1976)
Distribution Canada Coastal British Columbia to about 50º N United States N Washington to S California, west of the Cascades - Sierra Nevada axis
Habitat Open forests, rocky slopes, ravines, foothills, and shores; 0–1800 m.
USDA Hardiness Zone 7-9
RHS Hardiness Rating H4
Conservation status Least concern (LC)
This is the Arbutus of North America’s Pacific seaboard, potentially (if rarely) a magnificent large tree, one of the tallest members of the Ericaceae, rivalling the biggest Rhododendron species. Its attractions are clear: orange-red mature bark peeling in strips to reveal younger green layers beneath, copious scented white flowers in spring, and trusses of pea-sized orange or red fruits in autumn, often resembling bunches of grapes. Sometimes grown as a yard tree in its native range, it is notoriously difficult to transplant; further, it is an ‘active and messy tree’ (Arno & Hammerly 1977), shedding material for a significant part of the year, whether dead leaves, flowers, fruit or bark strips. There are better strawberry trees for the tidy, smaller garden. As a wild tree, however, the sight of multistemmed specimens slanting out over the edges of sea inlets and rocky shores on the Washington coast, or a brief glimpse of a thick-trunked giant while driving in the Coast Ranges of Northern California can be unforgettable. The temptation to capture something of this spirit in the garden is strong: to plant Arbutus menziesii is a statement of horticultural optimism.
The Pacific Madrone was known to early European explorers in California. The Spanish-born Franciscan missionary Joan Crespi, who chronicled the 1769–70 Portolá expedition, is usually credited with being the first to apply the name madroño, by analogy with the Old World A. unedo (McDonald & Tappeiner 1990). It was described scientifically (Pursh 1814) from specimens collected further north by Archibald Menzies, naturalist on George Vancouver’s HMS Discovery expedition of 1791–5. Seed was introduced from the Pacific Northwest to European cultivation in 1827 by another Scot, David Douglas (Bean 1976).
Arbutus menziesii is adapted to the mild, oceanic climate of the Pacific Northwest, yet has no absolute requirement for high rainfall, and is most abundant on well drained, rocky sites. It is most widespread in the lowlands west of the Cascades in Washington and Oregon, extending north to the Seymour Narrows area of British Columbia, becoming Canada’s only indigenous evergreen broadleaved tree, and south along the higher-rainfall western slopes of the Californian Coast Ranges, with outliers on the moister western flank of the Sierra Nevada (Arno & Hammerly 1977, McDonald & Tappeiner 1990). Records south of the Mexican border in Baja California are not supported by specimens, and are probably the result of the name madroño being applied locally to some Arctostaphylos species (Vizcaino & Minnich 1998).
Despite this distibution, it shows remarkable drought tolerance. Trees on California’s central coast showed little evidence of water stress during the exceptional drought of 2011–2015 (Chacon et al. 2020). A semi-sclerophyllous plant, its leaves become denser and narrower on dry, nutrient poor soils (Witkowski & Lamont 1991). Fine roots are able to penetrate cracks as narrow as 0.1 mm, where the roots become flattened although the central cylinder of vascular tissue is not deformed (Zwieniecki & Newton 1995), possibly aiding stability as well as water uptake. In a Seattle park, a natural Madrone stand on a rocky slope was shown to be important in stabilization, suggesting its planting for erosion control in such situations (Parker & Hamilton 1999). Open habitats are the norm. Even in woodland, where it is particularly associated with Pseudotsuga menziesii (also with Quercus garryana in Oregon’s Willamette Valley) it is found in more open areas (Arno & Hammerly 1977): this is a shade-intolerant tree.
In recent decades, a decline in the health of many madrones across the natural range has been noted, especially – but not exclusively – in larger urban specimens: branch dieback and tree death seem to have become more prevalent (Adams & Hamilton 1999). While no single cause has been identified, many fungi and Phytophthora spp. attack A. menziesii (Elliot 1999, Bennett & Shaw 2008); both environmental change and the rigours of urban life may increase susceptibility. Phytophthora cactorum causing root-rot especially with irrigation, Neofusicoccum arbuti (Nattrassia mangiferae) causing a canker on branches following damage, and Botryosphaeria dothidea (Fusicoccum aesculi) causing twig dieback, seem particularly important. Leaf spotting and necrosis caused by various fungi occurs under humid or shady conditions, both in Oregon (Bennett & Shaw 2008) and in European cultivation (J. Grimshaw pers. comm. 2020).
Pacific Madrone wood is cream or pinkish brown in colour, susceptible to decay and to warping on drying. It is valued as a veneer, is sometimes used in furniture, but is generally little-utilized and not managed by foresters (Meier 2021, McDonald & Tappeiner 1990). Like other Arbutus, it makes good firewood and charcoal; indeed, it was the choice of early settlers in California for producing the charcoal component of gunpowder (McDonald & Tappeiner 1990, Arno & Hammerly 1977). Various Native American peoples used bark infusions medicinally, on cuts and against various digestive, respiratory and skin ailments (Moerman 2021): some of these uses were still current on Vancouver Island in the late 20th century (Turner & Hebda 1990). It has also been used in tanning, and there are reports of Karok children using the exfoliating strips to make sledges (Moerman 2021). The mechanism of exfoliation remains unknown, but it may have some significance in reducing the load of epiphytic and climbing plants on the branches (Cabal et al. 2020).
The showy flowers are white, sometimes pinkish, but there seems to have been no horticultural selection for colour. As with other Arbutus species, bees are the main pollinators; there is at least some self-compatibility, with significant levels of self-fertilization in some inbred Canadian populations (Beland et al. 2005). The fruits are notably smaller than those of A. unedo, but with the potential for far more in each infructescence: a heavily fruiting tree is highly ornamental. Some wild trees seem to carry bigger crops than others, annually or biennially (Gonzalez 1999); it is unclear whether there is a genetic component to this, but if there were, good clones or strains would be worth selecting. Seeds are dispersed when the fleshy fruits are eaten by animals: birds, especially various pigeon species, are important, along with deer and rodents which take fallen fruit (McDonald & Tappeiner 1990). Indigenous people have eaten the fruit fresh, roasted or dried, as well using them for ‘cider’, not necessarily fermented (Moerman 2021). Reports of narcotic properties (Arno & Hammerly 1977) probably relate only to natural fermentation in the pulp, rather than to some more specific drug.
Arbutus menziesii has been planted as an ornamental widely, if not very commonly, across its native range. It is represented in arboreta from Vancouver to Berkeley, with a good number dating from the 1920s at Hoyt Arboretum, Portland, OR (University of British Columbia 2021, University of California Botanical Garden 2021, Hoyt Arboretum 2021). Sometimes also grown as a street tree, there is a handsome example planted in 1957 in southeast Portland (12.5m × 220 cm – Portland Parks & Recreation 2021). None of these yet rival the largest wild trees, some of which attract names and almost cult status. such as the Council Madrone of Humboldt Co., California, which before its death following a wet snow event was measured at 30.5 m tall with a crown spread of 35 m (Callahan 2015). It is said to have been a site of Native American council meetings (Coe 1983). The current US champion in Douglas Co., OR, was measured at 29 m × 635 cm, crown spread 30 m, in 2012 (American Forests 2021). It is probably winter cold that excludes this species from collections in the northeastern and central United States. However, the ever-experimental Denver Botanic Gardens, CO, have a young specimen on the rock garden, planted in 2005, showing what might become possible with clever siting (Denver Botanic Gardens 2021).
In European cultivation, it is probably again winter cold that restricts Pacific Madrone to the Atlantic fringe and the Low Countries. Good specimens are scattered across the British Isles, in both high and lower rainfall areas (The Tree Register 2021). Examples include trees at Hergest Croft, Herefordshire (24 m × 420 cm, 2013), Rowden Park, Frankby, Wirral (20 m × 440 cm, 2017 – probably planted in 1868) and Dundee University Botanic Garden (14 m × 258 cm, 2017 – planted in just 1974). Most appropriately, a fine old tree grows at Castle Menzies in Perthshire, Scotland, close to where Archibald Menzies was born and where he worked in the gardens as a boy (Rodger, Stokes & Ogilvie 2006). Few are of known wild provenance, but northerly material from British Columbia (EHOC 37 of 2016) has recently been planted in Scotland at Benmore, Logan and RBG Edinburgh (Royal Botanic Garden Edinburgh 2021). In Belgium, Arboretum Kalmthout has a 2004 accession (Plantcol 2021), while monumentaltrees.com (2021) records a lovely young, narrow-crowned specimen flowering well in an urban setting in Antwerp. The species is recorded at Bonn University Botanic Gardens (Botanische Gärten der Universität Bonn 2021), but it is unclear to what extent it is protected.
Propagation has usually been from seed, but this species is notorious for being difficult to transplant (Bean 1976). Root disturbance seems to be the problem, and conventional field-growing is not suitable on the nursery; careful, early planting of container-grown seedlings is appropriate and airpots would be ideal. Gonzalez (1999) describes a tested method for propagating nursery stock. Fruit should be collected only when fully ripe: the seeds should be dark brown to black. Seeds are cleaned, by first soaking the fruit in water for a week, then macerating and passing through a wire mesh which catches much of the fibrous pulp, but allows the small seeds to pass through; water and fine pulp is decanted off. The seeds are mixed with moist compost in a sealed bag and stratified for 60 days in a refrigerator, then sown in seed trays in a greenhouse at 15–18 °C: germination should begin in about a fortnight. Seedlings are pricked out into 10 cm pots of well drained compost at the 2–4 true leaf stage. McDonald & Tappeiner (1990) mention (sadly without reference) that propagation by cuttings is possible. While there is an extensive literature on mycorrhizal associations involving this species in the wild, their role in cultivation seems not to have been investigated.